|
For The Agency | For The Technology Developer | For The Magazine Publisher | For The Individual |
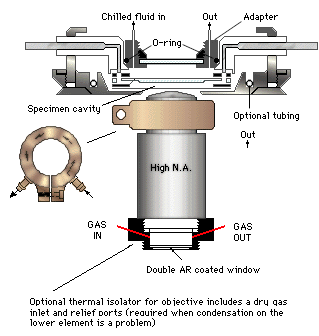
Studying Living CellsIt's not always easy, but studying living cells is how to find out what makes them tick The following is a manuscript for an article published in R&D magazine. R&D magazine holds the copyright for the finished article. C.G. Masi, Contributing Editor If you want something easy to image in a microscope, pick something that is dead. With dead things, you have plenty of time to set up your lighting, fiddle with positioning, add stains and so forth. The problem is that studying dead things tells you very little. You can observe their structure. You can sometimes learn about the channels they used to take up stains just before they died. That, however, is pretty much it. Mostly, you are looking at morphology. While morphology and structure are important things to know about cells, it doesn't tell you much about how they work. To answer many of the important questions in microbiology, you have to see cells at work. That means observing living cells. In Cells: A Laboratory Manual, Spector et al list two motivations for studying with live cells. Some studies attempt to establish natural behavior of cells as part of a living organism. Others attempt to determine changes brought about by adjusting a controlling factor. Obviously, both types of study are important to advancing our understanding of the genetic and protein mechanisms of living organisms--in both normal and diseased states--and for developing intervention strategies. What makes live cell microscopy difficult is that researchers have to consider the needs of their subjects as well as the needs of their instruments. Live cells, even differentiated cells from complex organisms, are living organisms themselves. They need to be fed, watered and sheltered if they are to perform as expected throughout the experiment. A live cell dropped on a slide under a microscope is equivalent to a human staked out in the desert. It won't function normally and it won't function long. While providing something close to normal care and feeding for your microscopic subjects is one aspect of the live cell problem, being able to see the things is another. Living cells are not designed to provide good optical contrast for microscopists, which is why stains are such an important part of cell-morphology studies. Most of the traditional stains, however, can prove more or less toxic to your experimental subjects. Thus, your choices for contrast media are much more limited when studying live cells. Finally, live cells move. Flagella flail. Pseudopods reach out. Organelles run chemical processes. Cytostructures bend and flex. Whole cells scurry about. External and internal movement is the hallmark of life, and it is these movements that we want to study. Care and Feeding of Live Cells Just as whole-organism researchers have developed enclosures to maintain their experimental subjects, microscopists have developed a wide range of microenvironmental chambers to contain and maintain live cells. These range from simple open petri dishes to continuous-flow perfusion chambers capable of tightly controlling the cells' physical and chemical environment for indefinite periods. The old drop of pond water on a glass slide maybe fine for some studies, but the water quickly heats up under the microscope light, and evaporates (which rapidly changes its chemistry). The limited fluid volume means that essential dissolved gases are used rapidly, while wastes from cellular metabolism have nowhere to go. Putting the drop in a well slide and adding a cover slip helps by preventing evaporation, but it also leads to faster heat build up. It also stops the only hope cells have of respiration. It's like putting a plastic bag over the head of the human you staked out in the desert. Fig. 1 shows the structure of an FCS2 closed-system cooled chamber available from Bioptechs in Butler, Penn. Cells are plated on a 40-mm coverslip and placed into the chamber, which provides a perfuseable laminar-flow optical chamber with user-modifiable flow characteristics. The upper glass element (microaqueduct slide) is then used to remove heat from the specimen cavity. The heat is absorbed in a cooled fluid being circulated through the cavity formed by the addition of an O-ring sealed window adapter. The cells remain safely enclosed in the separate optical enclosure. A separate ring is used to cool the microscope's objective lens if the study is to be done at below ambient temperature.
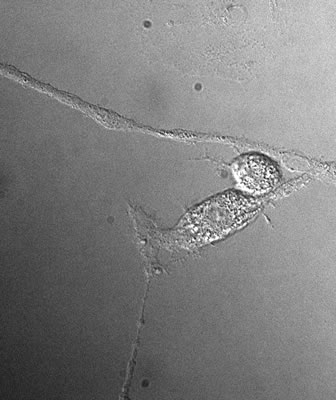
Developing Contrast Contrast in any optical system can be obtained by manipulating the intensity, color and phase of light as it passes through or reflects from a target. This applies to living cells as well as anything else. Most of the contrast we see in macroscopic systems comes from manipulating either the color or intensity (more often both) of the light we see with. Living cells, however, generally absorb very little light and have little or no color. That is why stains are so important to cell-morphology studies. The internal structures of living cells, however, affect the speed of light as it passes through them. That leads to the possibility of using phase contrast to image them (See Fig. 4). There are several phase contrast techniques available, such as differential interference contrast (DIC) and Nomarski DIC.
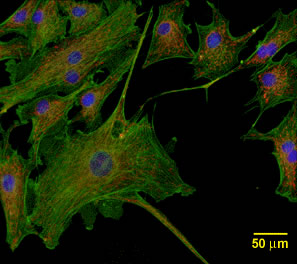
Phase contrast exploits one physical phenomenon: indices of refraction differ in the different materials making up the cell. It would be useful to have a contrast mechanism that keys on cellular chemistry, which is what interests researchers most. Fluorescence is such a contrast mechanism. Many biologically important molecules fluoresce. That is, they absorb light photons of one wavelength, hold the energy for a short time, and then expel that excess energy by reemitting another photon of a longer wavelength. By illuminating cells containing fluorescent molecules with the light at the appropriate excitation wavelength and observing them through a filter that isolates the emission wavelength, researchers have long exploited this phenomenon to obtain microscopic image contrast. Examples include fluorescent phalloidin for imaging actin filaments, fluorescent phospholipids for imaging plasma membranes, fluorescent ceramide for imaging Golgi apparati, and many others. This fluorescence technique has the decided advantage that it generally provides contrast without disturbing the normal operation of the cells' biochemical processes. The disadvantage, of course, is that natural fluorescence is largely serendipitous--at least for microscopy. It would be useful to have a fluorescent molecule available that could be used to tag various cellular chemical components more or less at will. Aequorea victoria is a luminescent jellyfish whose bioluminescence machinery includes a photoprotein, aequorin, which is activated by Ca++ ions and emits blue light, along with an accessory green fluorescent protein (commonly referred to simply as "GFP"), which accepts energy from aequorin and sheds it by emitting green light. Genetically modified GFP expressed in transgenic plants, fungi and animals has proven to be useful as a safe and bright fluorescent marker (see Fig. 2). Genes for producing GFP can be fused with the entire coding region of a particular protein, making it possible to follow functional, full-length proteins as they are used by living cells. Since GFP-tagged proteins can be followed in living cells, it is now possible to study dynamic systems.
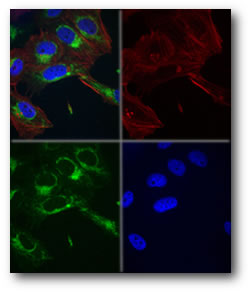
Imaging Cellular Motions Motions of live cells present two challenges to microscopists: obtaining clear images in the face of rapid cellular motions, and capturing the temporal development of cellular processes. Incorporation of digital video technology helps researchers meet both challenges. CCD cameras are roughly two orders of magnitude more sensitive than photographic films. This higher sensitivity allows microscopists to capture images with simultaneously lower illumination levels and shorter exposures. Motion-induced blur is proportional to speed of movement across the image divided by the exposure time. Thus, CCD cameras' increased sensitivity allows them to "freeze" cellular motions much more successfully in still images. To follow dynamic cellular processes, microscopists employ variable-frame-rate video. The limiting factor in CCD technology is frame-transfer rate, so, researchers generally have to off between speed and resolution. High-speed systems are available that can capture up to 100 frames per second, but their resolution is limited to roughly a quarter of a million pixels. Megapixel-resolution cameras are, of course, available, but they are limited to frame rates of 10 frames per second. Employing digital video technology also brings with it the advantages of image processing. Images can easily be combined (as shown in Fig. 3), enhanced and published electronically.
These advances in specimen chambers, contrast methods and digital imaging are making live cell microscopy an increasingly important part of the biotechnologist's tool kit. REFERENCE Spector, David L., Goldman, Robert D., and Leinwand, Leslie A., Cells: A Laboratory Manual,Cold Spring Harbor Laboratory Press, Plainview, N.Y., ISBN0-87969-522-6, 1998. WEBSITES |
|
Home | About Us | Technology Journalism | Technology Trends Library | Online Resources | Contact Us For The Agency | For The Technology Developer | For The Magazine Publisher | For The Individual © , C. G. Masi Technology Communications, Privacy Policy P.O. Box 10640, 978 S. San Pedro Road, Golden Valley, AZ 86413, USA Phone: +1 928.565.4514, Fax: +1 928.565.4533, Email: cgmasi@cgmasi.com, Web: www.cgmasi.com Developed by Telesian Technology Inc. |